 您的购物车当前为空
您的购物车当前为空
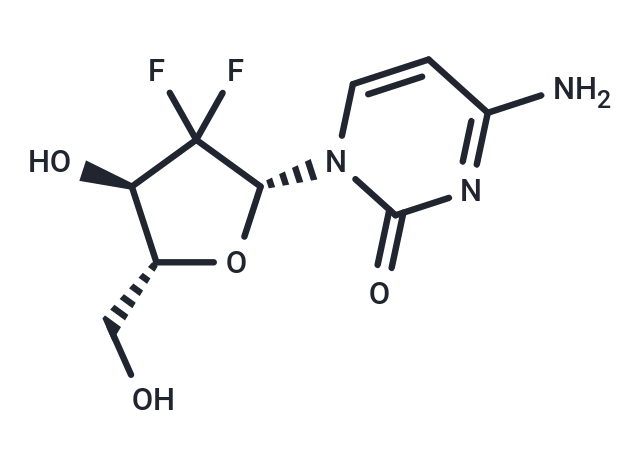
Gemcitabine
一键复制产品信息别名 吉西他滨, NSC 613327, LY188011
Gemcitabine (LY188011) 是一种人工合成的胞嘧啶核苷衍生物,一种 DNA 合成抑制剂。Gemcitabine 具有抗肿瘤活性和抗代谢活性。Gemcitabine 可以引起细胞自噬和凋亡。
Gemcitabine
一键复制产品信息Gemcitabine (LY188011) 是一种人工合成的胞嘧啶核苷衍生物,一种 DNA 合成抑制剂。Gemcitabine 具有抗肿瘤活性和抗代谢活性。Gemcitabine 可以引起细胞自噬和凋亡。
| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5 mg | ¥ 147 | 现货 | |
| 10 mg | ¥ 195 | 现货 | |
| 25 mg | ¥ 298 | 现货 | |
| 50 mg | ¥ 423 | 现货 | |
| 100 mg | ¥ 592 | 现货 | |
| 500 mg | ¥ 1,680 | 现货 | |
| 1 mL x 10 mM (in DMSO) | ¥ 133 | 现货 |
产品介绍
| 产品描述 | Gemcitabine (LY188011) is a synthetic cytosine nucleoside derivative and an inhibitor of DNA synthesis. Gemcitabine has antitumor and antimetabolic activities. Gemcitabine induces autophagy and apoptosis. |
| 靶点活性 | BxPC-3 cells:18nM, MIAPaCa2 cells:40 nM, Capan-2 cells:12 nM |
| 体外活性 | 方法:PDAC 衍生成对原发性癌症细胞 (PCCs) PCC-1、PCC-2、PCC-5、PCC-6 和 PDAC 细胞 BxPC-3、 Mia PaCa-2、Panc-1 用 Gemcitabine (0.001-1000 µM) 处理 48 h,使用 MTT 方法检测细胞生长抑制情况。 |
| 体内活性 | 方法:为检测体内抗肿瘤活性,将 Gemcitabine (20 mg/kg) 腹腔注射给携带人高级别脑膜瘤肿瘤 HKBMM 的 BALB/cAJcl-nu/nu 小鼠,每周两次,持续四周。 |
| 细胞实验 | The cytotoxic effect of gemcitabine was evaluated with the MTT assay. SPC-A1 or A549 cells were treated with gemcitabine (0.05–500 lM) for 24 h. Then, 10 ll of MTT (5 mg/ml in PBS) was added to each well and incubated for 4 h at 37 C. Then, the formazan crystals were solubilized with 200 ll DMSO. The absorbance at 570 nm was measured using an automatic multiwell spectrophotometer. The experiment was repeated four times for each group [3]. |
| 动物实验 | At 1 month of age, LSL-Kras G12D/+; LSL-Trp53 R172H; Pdx-1-Cre mice are randomized into treatment groups (placebo, DMAPT, Gemcitabine, DMAPT/Gemcitabine). Placebo (vehicle=hydroxylpropyl methylcellulose, 0.2% Tween 80 [HPMT]) and DMAPT (40 mg/kg body weight in HPMT) are administered by oral gastric lavage once daily. Gemcitabine (50 mg/kg body weight in PBS) is administered by intraperitoneal injection twice weekly. Mouse weight is monitored weekly. Treatment is continued until mice show signs of lethargy, abdominal distension or weight loss at which time they are sacrificed. Successful excision-recombination events are confirmed in the pancreata of mice by detecting the presence of a single LoxP site [5]. |
| 别名 | 吉西他滨, NSC 613327, LY188011 |
| 分子量 | 263.20 |
| 分子式 | C9H11F2N3O4 |
| CAS No. | 95058-81-4 |
| Smiles | NC1=NC(=O)N(C=C1)[C@@H]1O[C@H](CO)[C@@H](O)C1(F)F |
| 密度 | 1.84 g/cm3 (Predicted) |
| 存储 | keep away from direct sunlight,store at low temperature | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | |||||||||||||||||||||||||
| 溶解度信息 | H2O: 6.25 mg/mL (23.75 mM), Sonication is recommended. DMSO: 125.00 mg/mL (474.92 mM), Sonication is recommended. Ethanol: 12.50 mg/mL (47.49 mM), Sonication is recommended. | |||||||||||||||||||||||||
| 体内实验配方 | 5% DMSO+95% Saline: 0.75 mg/mL (2.85 mM), Solution. 请按顺序添加溶剂,在添加下一种溶剂之前,尽可能使溶液澄清。如有必要,可通过加热、超声、涡旋处理进行溶解。工作液建议现配现用。以上配方仅供参考,体内配方并不是绝对的,请根据不同情况进行调整。 | |||||||||||||||||||||||||
溶液配制表 | ||||||||||||||||||||||||||
H2O/Ethanol/DMSO
| ||||||||||||||||||||||||||
 化合物与蛋白结合的复合物
化合物与蛋白结合的复合物
Drosophila melanogaster deoxyribonucleoside kinase successfully activates gemcitabine in transduced cancer cell lines
计算器
体内实验配液计算器
以上为“体内实验配液计算器”的使用方法举例,并不是具体某个化合物的推荐配制方式,请根据您的实验动物和给药方式选择适当的溶解方案。
剂量转换
对于不同动物的给药剂量换算,您也可以参考 更多




 很棒
很棒
 |
|