 您的购物车当前为空
您的购物车当前为空
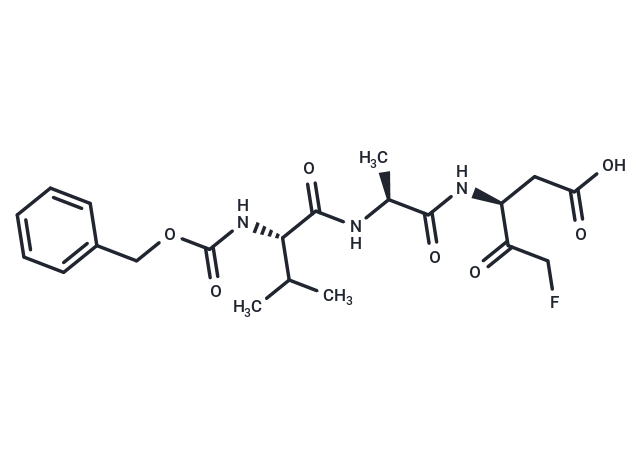
Z-VAD-FMK
一键复制产品信息别名 Z-VAD(OH)-FMK, Caspase Inhibitor VI
Z-VAD-FMK (Caspase Inhibitor VI) 是一种 caspase 的广谱抑制剂。Z-VAD-FMK 可以与活化的 caspase 结合,从而抑制细胞凋亡。Z-VAD-FMK即使在高达 440μM 的浓度下也不会抑制 UCHL1 的活性。
Z-VAD-FMK
一键复制产品信息Z-VAD-FMK (Caspase Inhibitor VI) 是一种 caspase 的广谱抑制剂。Z-VAD-FMK 可以与活化的 caspase 结合,从而抑制细胞凋亡。Z-VAD-FMK即使在高达 440μM 的浓度下也不会抑制 UCHL1 的活性。
| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1 mg | ¥ 698 | 现货 | |
| 5 mg | ¥ 2,550 | 现货 | |
| 10 mg | ¥ 3,170 | 现货 | |
| 25 mg | ¥ 5,200 | 现货 | |
| 50 mg | ¥ 7,350 | 现货 | |
| 100 mg | ¥ 9,890 | 现货 | |
| 200 mg | ¥ 13,300 | 现货 | |
| 1 mL x 10 mM (in DMSO) | ¥ 2,550 | 现货 |
产品介绍
| 产品描述 | Z-VAD-FMK (Caspase Inhibitor VI) is a broad-spectrum inhibitor of caspases.Z-VAD-FMK binds to activated caspases and inhibits apoptosis.Z-VAD-FMK does not inhibit UCHL1 activity, even at concentrations up to 440 μM. |
| 靶点活性 | Parasite growth:2.7 μM |
| 体外活性 | 方法:中性粒细胞用 Z-VAD-FMK (0.03-300 µM) 处理 30 min,再加入在加入 200 U/mL TNFα 孵育 6 h,使用 Flow Cytometry 方法检测细胞凋亡情况。 |
| 体内活性 | 方法:为检测体内抗肿瘤活性,用 RT (2 Gy 局部照射肿瘤,第 8/9/10 天)、DTIC (2 mg/只腹腔注射,第 8/10 天)、将 Z-VAD-FMK (2 mg/kg 腹腔注射,第 8/9/10 天) 和 HT (放射后进行 4 h,第 8/10 天) 联合治疗携带小鼠黑色素瘤肿瘤 B16 的 C57/BL6 小鼠。 |
| 别名 | Z-VAD(OH)-FMK, Caspase Inhibitor VI |
| 分子量 | 453.46 |
| 分子式 | C21H28FN3O7 |
| CAS No. | 161401-82-7 |
| Smiles | CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)CF |
| 密度 | 1.260 g/cm3 (Predicted) |
| Sequence | Cbz-Val-Ala-Asp(OMe)-CH2F |
| Sequence Short | VAX |
| 存储 | store at low temperature,keep away from direct sunlight | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | |||||||||||||||||||||||||||||||||||
| 溶解度信息 | H2O: < 1 mg/mL (insoluble or slightly soluble) Ethanol: 83 mg/mL (183.04 mM), Sonication is recommended. DMSO: 247.5 mg/mL (545.8 mM), Sonication is recommended. | |||||||||||||||||||||||||||||||||||
| 体内实验配方 | 5% DMSO+95% Saline: 4.15 mg/mL (9.15 mM), Solution. 请按顺序添加溶剂,在添加下一种溶剂之前,尽可能使溶液澄清。如有必要,可通过加热、超声、涡旋处理进行溶解。工作液建议现配现用。以上配方仅供参考,体内配方并不是绝对的,请根据不同情况进行调整。 | |||||||||||||||||||||||||||||||||||
溶液配制表 | ||||||||||||||||||||||||||||||||||||
Ethanol/DMSO
| ||||||||||||||||||||||||||||||||||||
计算器
体内实验配液计算器
剂量转换
对于不同动物的给药剂量换算,您也可以参考 更多




 很棒
很棒
 |
|