 您的购物车当前为空
您的购物车当前为空
Z-VAD(OMe)-FMK
一键复制产品信息别名 Z-Val-Ala-Asp(OMe)-FMK, Z-VAD-FMK
Z-VAD(OMe)-FMK 是一种具有不可逆特性的pan-caspase抑制剂。Z-VAD(OMe)-FMK 还是泛素 C 端水解酶 L1 (UCHL1) 的抑制剂,它通过靶向 UCHL1 活性位点进行不可逆修饰。
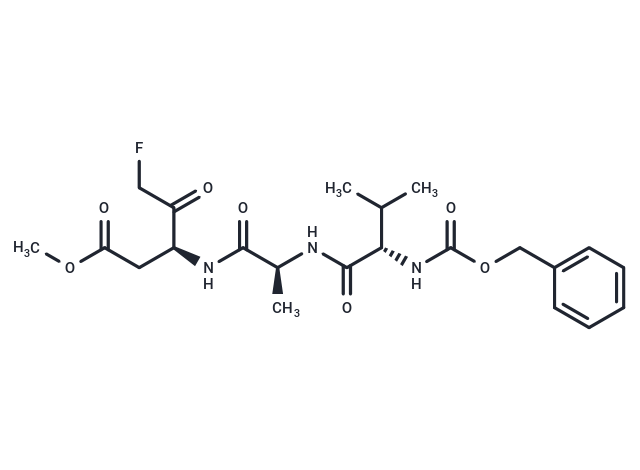
Z-VAD(OMe)-FMK
一键复制产品信息Z-VAD(OMe)-FMK 是一种具有不可逆特性的pan-caspase抑制剂。Z-VAD(OMe)-FMK 还是泛素 C 端水解酶 L1 (UCHL1) 的抑制剂,它通过靶向 UCHL1 活性位点进行不可逆修饰。
| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1 mg | ¥ 538 | 现货 | |
| 5 mg | ¥ 1,390 | 现货 | |
| 10 mg | ¥ 2,230 | 现货 | |
| 25 mg | ¥ 3,570 | 现货 | |
| 50 mg | ¥ 4,890 | 现货 | |
| 100 mg | ¥ 6,790 | 现货 | |
| 1 mL x 10 mM (in DMSO) | ¥ 1,450 | 现货 |
产品介绍
| 产品描述 | Z-VAD(OMe)-FMK is a pan-caspase inhibitor with irreversible properties; Z-VAD(OMe)-FMK is also an inhibitor of ubiquitin C terminal hydrolase L1 (UCHL1), which is irreversibly modified by targeting the UCHL1 active site. |
| 靶点活性 | SARS-CoV-2 Mpro:0.59 μM |
| 体外活性 | 方法:人白血病细胞 HL60 用 Z-VAD(OMe)-FMK (50 µM) 和 camptothecin (150 μM) 处理 3 h,使用电子显微镜观察细胞形态。 |
| 体内活性 | 方法:为研究 Z-VAD(OMe)-FMK 的体内给药是否能预防感染诱导的早产,将 Z-VAD(OMe)-FMK (10 mg/kg) 单次腹腔注射给用热致死的 B 组链球菌 (HK-GBS) 诱导早产的 CD1 小鼠。 |
| 细胞实验 | The human monocytic tumour cell line, THP.1 and the leukaemic T-cell line, Jurkat (clone E-6) were maintained in RPMI 1640 supplemented with 10% (v/v) heat-inactivated fetal calf serum, 100 units/ml penicillin and 100 μg/ml streptomycin in an atmosphere of 5% CO2 in air at 37 °C. The cells were maintained in logarithmic growth phase by routine passage every 2–3 days. To induce apoptosis in THP.1 cells, 2×10^6 cells/ml were incubated either alone or in the presence of cycloheximide (25 μM) and TLCK (100 μM) as previously described. In order to assess the possible effects of various ICE-like protease inhibitors, THP.1 cells were also pretreated for 1 h with Z-VAD.FMK (10 μM), Ac-DEVD-CHO (20 μM) and Ac-YVAD-CHO (20 μM) before being exposed to the apoptotic stimulus. To induce apoptosis in Jurkat cells, 2×10^6 cells/ml were stimulated with 200 ng/ml anti-human Fas as described previously [1]. |
| 动物实验 | Mice used in this study were 5- to 6-week-old (20 to 22 g) ICR males. Mice were injected with 30 mg/kg LPS from E. coli serotype O111:B4 through the tail vein. Z-VAD.fmk was dissolved at 2 mg/ml in 1% dimethyl sulfoxide in sterile saline, and administered to mice by the method of Rodriguez et al. A single intravenous injection of Z-VAD.fmk (0.25 mg) was made 15 minutes before LPS injection, followed by three intravenous injections of Z-VAD.fmk (0.1 mg each) per hour. Control mice were injected with the same volume of 1% DMSO in sterile saline [4]. |
| 别名 | Z-Val-Ala-Asp(OMe)-FMK, Z-VAD-FMK |
| 分子量 | 467.49 |
| 分子式 | C22H30FN3O7 |
| CAS No. | 187389-52-2 |
| Smiles | COC(=O)C[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C)C(=O)CF |
| 密度 | 1.214g/cm3 |
| Sequence | Z-Val-Ala-Asp(OMe)-FMK |
| Sequence Short | ZVA-D(OMe)-FMK |
| 存储 | store at low temperature,keep away from direct sunlight | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | |||||||||||||||||||||||||||||||||||
| 溶解度信息 | H2O: < 1 mg/mL (insoluble or slightly soluble) Ethanol: < 1 mg/mL (insoluble or slightly soluble) DMSO: 126.25 mg/mL (270.06 mM), Sonication is recommended. | |||||||||||||||||||||||||||||||||||
| 体内实验配方 | 10% DMSO+40% PEG300+5% Tween 80+45% Saline: 4.5 mg/mL (9.63 mM), Solution. 请按顺序添加溶剂,在添加下一种溶剂之前,尽可能使溶液澄清。如有必要,可通过加热、超声、涡旋处理进行溶解。工作液建议现配现用。以上配方仅供参考,体内配方并不是绝对的,请根据不同情况进行调整。 | |||||||||||||||||||||||||||||||||||
溶液配制表 | ||||||||||||||||||||||||||||||||||||
DMSO
| ||||||||||||||||||||||||||||||||||||
 化合物与蛋白结合的复合物
化合物与蛋白结合的复合物
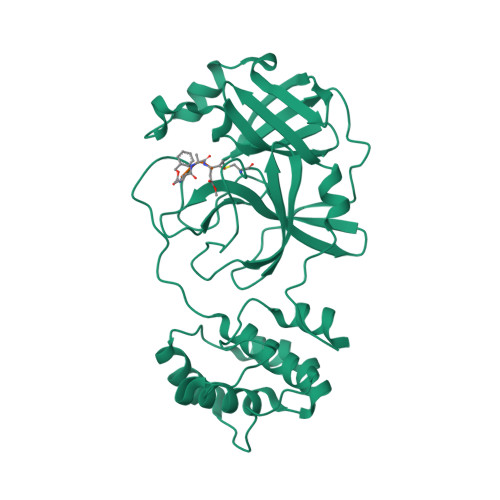
Crystal structure of the SARS-CoV-2 main protease in complex with Z-VAD(OMe)-FMK
计算器
体内实验配液计算器
剂量转换
对于不同动物的给药剂量换算,您也可以参考 更多




 很棒
很棒
 |
|