 您的购物车当前为空
您的购物车当前为空
Capivasertib
一键复制产品信息别名 AZD5363
Capivasertib (AZD5363) 是一种广谱的 AKT 抑制剂,对 Akt1、Akt2 和 Akt3 均有抑制活性 (IC50=3/7/7 nM),具有口服活性。Capivasertib 具有抗肿瘤活性,可以用于治疗乳腺癌。

为众多的药物研发团队赋能,
让新药发现更简单!
Capivasertib
一键复制产品信息Capivasertib (AZD5363) 是一种广谱的 AKT 抑制剂,对 Akt1、Akt2 和 Akt3 均有抑制活性 (IC50=3/7/7 nM),具有口服活性。Capivasertib 具有抗肿瘤活性,可以用于治疗乳腺癌。
| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1 mg | ¥ 329 | 现货 | |
| 2 mg | ¥ 473 | 现货 | |
| 5 mg | ¥ 787 | 现货 | |
| 10 mg | ¥ 1,160 | 现货 | |
| 25 mg | ¥ 1,850 | 现货 | |
| 50 mg | ¥ 2,650 | 现货 | |
| 100 mg | ¥ 3,930 | 现货 | |
| 200 mg | ¥ 5,590 | 现货 | |
| 1 mL x 10 mM (in DMSO) | ¥ 742 | 现货 |
产品介绍
| 产品描述 | Capivasertib (AZD5363) is a broad-spectrum AKT inhibitor with inhibitory activity against Akt1, Akt2, and Akt3 (IC50=3/7/7 nM) with oral activity. Capivasertib has antitumor activity for the treatment of breast cancer. |
| 靶点活性 | p70 S6K:6 nM (cell free), ROCK2:60 nM, Akt3:7 nM (cell free), ROCK1:470 nM, PKA:7 nM (cell free), Akt2:7 nM (cell free), Akt1:3 nM (cell free) |
| 体外活性 | 方法:六株人胃癌细胞用 Capivasertib (40 nM-50 µM) 处理 72 h,使用 SRB Assay 检测细胞活力。 |
| 体内活性 | 方法:为检测体内抗肿瘤活性,将 Capivasertib (100-300 mg/kg,10% DMSO 25% w/v Kleptose HPB buffer) 灌胃给药给携带乳腺癌肿瘤 BT474c 的 nude 小鼠,每天两次,持续两周。 |
| 激酶实验 | The ability of AZD5363 to inhibit the activity of AKT1, AKT2 and AKT3 was evaluated by the Caliper Off-Chip Incubation Mobility Shift Assay. Active recombinant AKT1, AKT2, or AKT3 were incubated with a 5-FAM-labeled custom-synthesized peptide substrate together with increasing concentrations of inhibitor. Final reactions contained 1 to 3 nmol/L AKT1, AKT2, or AKT3 enzymes; 1.5 μmol/L peptide substrate; ATP at Km for each AKT isoform; 10 mmol/L MgCl2, 4 mmol/L dithiothreitol (DTT), 100 mmol/L HEPES, and 0.015% Brij-35. The reactions were incubated at room temperature for 1 hour and stopped by the addition of a buffer containing 100 mmol/L HEPES, 0.015% Brij-35 solution, 0.1% coating reagent, 40 mmol/L EDTA, and 5% DMSO. Plates were then analyzed using a Caliper LC3000, allowing for separation of peptide substrate and phosphorylated product by electrophoresis with subsequent detection and quantification of laser-induced fluorescence. To determine the kinase selectivity profile, AZD5363 was also tested against PKA, ROCK1, ROCK2, and P70S6K. PKA, ROCK1, and ROCK2 activity were determined by Caliper Off-Chip Incubation Mobility Shift Assay, as described earlier. Final reaction conditions for measuring ROCKI activity were 5 nmol/L active recombinant ROCK1, 1.5 μmol/L fluorescein isothiocyanate (FITC)-labeled custom peptide substrate, 7 μmol/L ATP, 1 mmol/L DTT, 5 mmol/L MgCl2, 100 mmol/L HEPES, 0.015% Brij-35, and 5 mmol/L β-glycerophosphate; final reaction for measuring ROCK2 activity contained 7.5 nmol/L active recombinant ROCK2, 1.5 μmol/L FAM-labeled custom peptide substrate, 7.5 μmol/L ATP, 1 mmol/L DTT, 10 mmol/L MgCl2, 100 mmol/L HEPES, 0.015% Brij-35, and 5 mmol/L β-glycerophosphate; and protein kinase A (PKA) activity was measured in a final reaction containing 0.0625 nmol/L PKA, 3 μmol/L FITC-labeled custom peptide substrate, 4.6 μmol/L ATP, 1 mmol/L DTT, 10 mmol/L MgCl2, 110 mmol/L HEPES, and 0.015% Brij-35.P70S6K activity was measured by a radioactive (33P-ATP) filter-binding assay. Recombinant S6K1 (T412E) was assayed against a substrate peptide (KKRNRTLTV) in a final volume of 25.5 μL containing 8 mmol/L MOPS, 200 μmol/L EDTA, 100 μmol/L substrate peptide, 10 mmol/L magnesium acetate, 20 μmol/L γ-33P-ATP (50–1,000 cpm/pmol), and increasing concentrations of AZD5363. The reactions were incubated for 30 minutes at room temperature and terminated by the addition of 0.5 mol (3%) orthophosphoric acid. Reactions were then harvested onto a P81 UniFilter and product formation quantified. IC50 values for all enzyme assays were obtained by fitting data in Origin 7.0. |
| 细胞实验 | A high-throughput screening cell-based assay was developed to measure cellular AKT activity using the MDA-MB-468 breast cancer cell line. Cells were exposed to AZD5363 at concentrations ranging from 3 to 0.003 μmol/L. After a 2-hour treatment, cells were fixed with formaldehyde, washed, permeabilized with 0.5% polysorbate 20 and then probed with a phospho-specific antibody against GSK3βser9. Levels of phosphorylated GSK3βser9 were measured with an Acumen Explorer laser scanning cytometer and IC50 values estimates by fitting data in Origin 7.0. |
| 动物实验 | When mean tumor sizes reached approximately 0.2 cm^3, the mice were randomized into control and treatment groups. The treatment groups received varying dose schedules of AZD5363 solubilized in a 10% DMSO 25% w/v Kleptose HPB buffer by oral gavage, docetaxel solubilized in 2.6% ethanol in injectable water by intravenous injection once on day 1 at 15 or 5 mg/kg once weekly. When administered in combination, docetaxel was administered 1 hour before the oral dose of AZD5363. The control group received the DMSO/Kleptose buffer alone, twice daily by oral gavage. Tumor volumes (measured by caliper), animal body weight, and tumor condition were recorded twice weekly for the duration of the study. Mice were sacrificed by CO2 euthanasia. The tumor volume was calculated (taking length to be the longest diameter across the tumor and width to be the corresponding perpendicular diameter) using the formula: (length × width) × √(length × width) × (π/6). Growth inhibition from the start of treatment was assessed by comparison of the differences in tumor volume between control and treated groups. Because the variance in mean tumor volume data increases proportionally with volume (and is therefore disproportionate between groups), data were log transformed to remove any size dependency before statistical evaluation. Statistical significance was evaluated using a one-tailed, 2-sample t-test. |
| 别名 | AZD5363 |
| 分子量 | 428.92 |
| 分子式 | C21H25ClN6O2 |
| CAS No. | 1143532-39-1 |
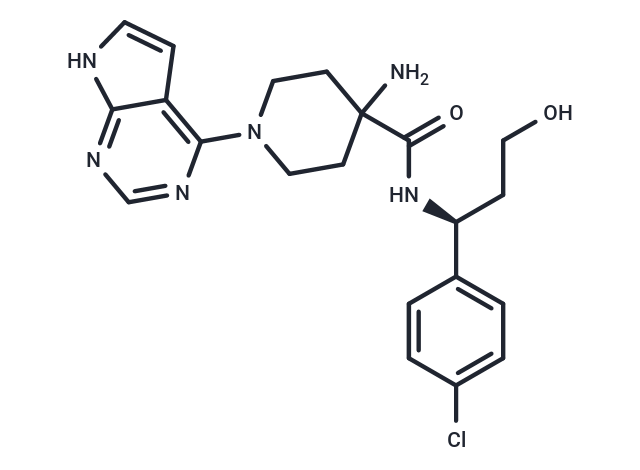
| Smiles | NC1(CCN(CC1)c1ncnc2[nH]ccc12)C(=O)N[C@@H](CCO)c1ccc(Cl)cc1 |
| 密度 | 1.381 g/cm3 |
| 颜色 | White |
| 物理性状 | Solid |
| 存储 | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | ||||||||||||||||||||||||||||||||||||||||
| 溶解度信息 | Ethanol: 1 mg/mL (2.33 mM), Sonication is recommended. DMSO: 130 mg/mL (303.09 mM), Sonication is recommended. H2O: Insoluble | ||||||||||||||||||||||||||||||||||||||||
| 体内实验配方 | 10% DMSO+40% PEG300+5% Tween 80+45% Saline: 8 mg/mL (18.65 mM), Solution. 请按顺序添加溶剂,在添加下一种溶剂之前,尽可能使溶液澄清。如有必要,可通过加热、超声、涡旋处理进行溶解。工作液建议现配现用。以上配方仅供参考,体内配方并不是绝对的,请根据不同情况进行调整。 | ||||||||||||||||||||||||||||||||||||||||
溶液配制表 | |||||||||||||||||||||||||||||||||||||||||
Ethanol/DMSO
DMSO
| |||||||||||||||||||||||||||||||||||||||||
计算器
体内实验配液计算器
以上为“体内实验配液计算器”的使用方法举例,并不是具体某个化合物的推荐配制方式,请根据您的实验动物和给药方式选择适当的溶解方案。
剂量转换
对于不同动物的给药剂量换算,您也可以参考 更多




 很棒
很棒
 |
|