购物车
 您的购物车当前为空
您的购物车当前为空
别名 瑞格列净乙酸酯, GSK-189075A, GSK189075A, GSK-189075, GSK189075, GSK 189075A, GSK 189075
Remogliflozin etabonate (GSK189075A) 是 regaliflozin 的前药和 SGLT2 抑制剂,对 hSGLT2、rSGLT2、rSGLT1 和 hSGLT1 的 Ki 值分别为 1.95、2.14、8.57 和 43.1μM。

为众多的药物研发团队赋能,
让新药发现更简单!
Remogliflozin etabonate (GSK189075A) 是 regaliflozin 的前药和 SGLT2 抑制剂,对 hSGLT2、rSGLT2、rSGLT1 和 hSGLT1 的 Ki 值分别为 1.95、2.14、8.57 和 43.1μM。
| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1 mg | ¥ 197 | 现货 | |
| 5 mg | ¥ 497 | 现货 | |
| 10 mg | ¥ 893 | 现货 | |
| 25 mg | ¥ 1,690 | 现货 | |
| 50 mg | ¥ 2,570 | 现货 | |
| 100 mg | ¥ 3,580 | 现货 | |
| 200 mg | ¥ 4,950 | 现货 | |
| 1 mL x 10 mM (in DMSO) | ¥ 572 | 现货 |
| 产品描述 | Remogliflozin etabonate (GSK189075A) is a prodrug of regaliflozin and an inhibitor SGLT2 with Ki values of 1.95, 2.14, 8.57, and 43.1μM for hSGLT2, rSGLT2, rSGLT1, and hSGLT1, respectively. |
| 靶点活性 | SGLT1 (rat):8.57 μM (Ki), SGLT2 (rat):2.14 μM (Ki), SGLT2 (human):1.95 μM (Ki), SGLT1 (human):43.1 μM (Ki) |
| 体内活性 | 口服Remogliflozin etabonate(10-30 mg/kg)能剂量依赖性地降低FPG和GHb水平。口服Remogliflozin etabonate(3-30 mg/kg)亦能剂量依赖性地增加尿量及尿糖排泄量[2]。 |
| 别名 | 瑞格列净乙酸酯, GSK-189075A, GSK189075A, GSK-189075, GSK189075, GSK 189075A, GSK 189075 |
| 分子量 | 522.59 |
| 分子式 | C26H38N2O9 |
| CAS No. | 442201-24-3 |
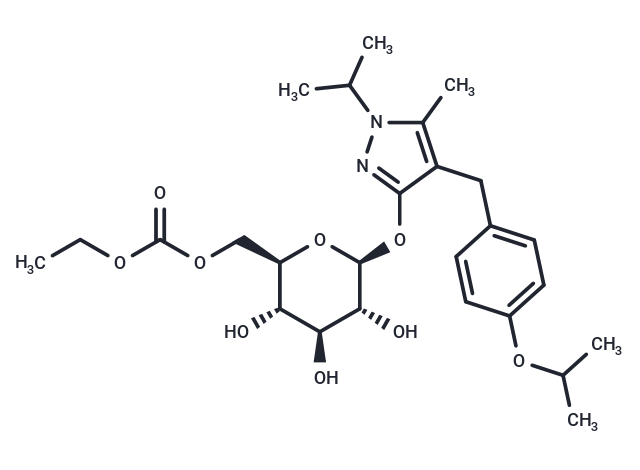
| Smiles | CCOC(=O)OC[C@H]1O[C@@H](Oc2nn(C(C)C)c(C)c2Cc2ccc(OC(C)C)cc2)[C@H](O)[C@@H](O)[C@@H]1O |
| 密度 | 1.31 g/cm3 (Predicted) |
| 存储 | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | |||||||||||||||||||||||||||||||||||
| 溶解度信息 | DMSO: 90 mg/mL (172.22 mM), Sonication is recommended. | |||||||||||||||||||||||||||||||||||
| 体内实验配方 | 10% DMSO+40% PEG300+5% Tween 80+45% Saline: 3.3 mg/mL (6.31 mM), Sonication is recommended. 请按顺序添加溶剂,在添加下一种溶剂之前,尽可能使溶液澄清。如有必要,可通过加热、超声、涡旋处理进行溶解。工作液建议现配现用。以上配方仅供参考,体内配方并不是绝对的,请根据不同情况进行调整。 | |||||||||||||||||||||||||||||||||||
溶液配制表 | ||||||||||||||||||||||||||||||||||||
DMSO
| ||||||||||||||||||||||||||||||||||||
对于不同动物的给药剂量换算,您也可以参考 更多