 您的购物车当前为空
您的购物车当前为空
MG-132
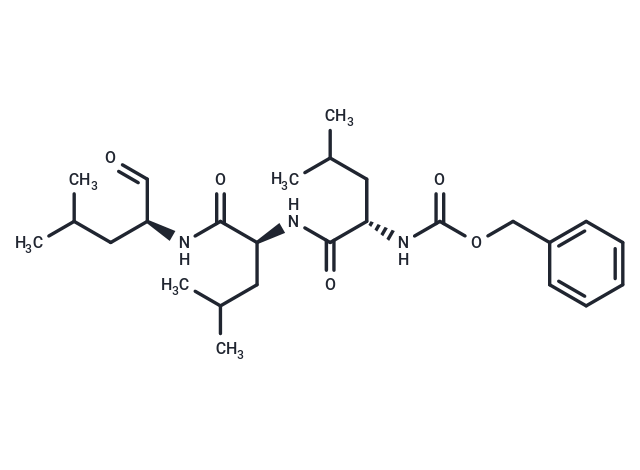
一键复制产品信息别名 Z-LLL-al, Z-Leu-Leu-Leu-CHO, MG132
MG-132 (Z-Leu-Leu-Leu-al) 是一种 26S 蛋白酶体抑制剂 (IC50=100 nM),具有细胞渗透性、可逆性。MG-132 可作为自噬激活剂,可诱导凋亡。

为众多的药物研发团队赋能,
让新药发现更简单!
MG-132
一键复制产品信息MG-132 (Z-Leu-Leu-Leu-al) 是一种 26S 蛋白酶体抑制剂 (IC50=100 nM),具有细胞渗透性、可逆性。MG-132 可作为自噬激活剂,可诱导凋亡。
| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1 mg | ¥ 137 | 现货 | |
| 5 mg | ¥ 276 | 现货 | |
| 10 mg | ¥ 382 | 现货 | |
| 25 mg | ¥ 828 | 现货 | |
| 50 mg | ¥ 1,280 | 现货 | |
| 100 mg | ¥ 1,850 | 现货 | |
| 200 mg | ¥ 2,580 | 现货 | |
| 500 mg | ¥ 4,330 | 现货 | |
| 1 mL x 10 mM (in DMSO) | ¥ 353 | 现货 |
产品介绍
| 产品描述 | MG-132 (Z-Leu-Leu-Leu-al) is a 26S proteasome inhibitor (IC50=100 nM) that is cell-permeable and reversible. MG-132 acts as an autophagy activator and also induces apoptosis. |
| 靶点活性 | Calpain:1.2 μM (cell free), COS-7 cells:< 10 μM, 20S proteasome:100 nM (cell free), HCT116 cells:0.82 μM |
| 体外活性 | 方法:人宫颈癌细胞 HeLa 用 MG-132 (0.5-30 μM) 处理 24 h,使用 MTT 方法检测细胞生长抑制情况。 |
| 体内活性 | 方法:为检测体内抗肿瘤活性,将 MG-132 (1 mg/kg) 静脉注射给携带人宫颈癌肿瘤 HeLa、CaSki 或 C33A 的 C.B‐17/lcr‐scid/scidJcl 小鼠,每周两次,持续四周。 |
| 激酶实验 | Inhibitory activities of ZLLa1 and ZLLLal against m-calpain and 20S proteasome were measured by previously described methods.For the m-calpain inhibitory assay,the 0.5 ml reaction mixture contained 0.24% alkali-denatured casein,28 mM 2-mercaptoethanol,0.94 unit of m-calpain,ZLLal or ZLLLal,6 mM CaCl2,and 0.1M Tris-HC1 (pH 7.5).The reaction was started by the addition of m-calpain solution and stopped by the addition of 0.5 ml of 10% trichloroacetic acid after incubation at 30℃ for 15 min.After centrifugation at 1,300×g for 10 min,the absorbance of the supernatant at 280 nm was measured.The reaction mixture for the 20S proteasome inhibitory assay contained 0.1 M Tris-acetate,pH 7.0,20S proteasome,ZLLa1 or ZLLLal,and 25 μM substrate dissolved in dimethyl sulfoxide in a final volume of 1 ml.After incubation at 37℃ for 15 min,the reaction was stopped by the addition of 0.1 ml of 10% SDS and 0.9 ml of 0.1 M Tris-acetate,pH 9.0.The fluorescence of the reaction products was measured.To determine the IC50s against m-calpain and 20S proteasome,various concentrations of the synthetic peptide aldehydes were included in the assay mixture [1]. |
| 细胞实验 | The effect of MG132 on HeLa cell growth was determined by trypan blue exclusion cell counting or measuring MTT dye absorbance of living cells as previously described. In brief, cells (5x10^5 cells per well) were seeded in 24-well plates for cell counting, and cells (5x10^4 cells per well) were seeded in 96-well microtiter plates for the MTT assay. After exposure to indicated amounts of MG132 for 24 h, cells in 24-well plates or 96-well plates were collected with trypsin digestion for trypan blue exclusion cell counting or were used for the MTT assay. Twenty microliters of MTT solution (2 mg/ml in PBS) was added to each well of 96-well plates. The plates were again incubated for 4 h at 37?C. MTT solution in the medium was aspirated off and 200 μl of DMSO was added to each well to solubilize the formazan crystals formed in viable cells. Optical density was measured at 570 nm using a microplate reader. Each plate contained multiple wells at a given experimental condition and multiple control wells. This procedure was replicated for 2-4 plates per condition [3]. |
| 动物实验 | Male Sprague–Dawley rats (8 weeks old, 180 – 230 g) were used to establish a pressure-overload model as described previously. All animals were separated into four groups (10 rats per group): (i) vehicle-treated sham group; (ii) MG132-treated sham group; (iii) vehicle-treated abdominal aortic banding (AAB) group; and (iv) MG132-treated AAB group. Under intraperitoneal pentobarbital (50 mg/kg) anesthesia, AAB was created using a 5-0 suture tied twice around the abdominal aorta in which. a 21-gauge needle was inserted. The needle was then retracted yielding a 70 – 80% constriction with an outer aortic diameter of 0.8 mm. In the sham surgery rats, the same surgery was performed as described above except the aorta was constricted. At Day 3 after the surgery, MG132-treated rats were intraperitoneally injected with 0.1 mg/kg/day of MG132 for 8 weeks. All control animals were injected with a corresponding volume of vehicle only (0.1% DMSO) [4]. Sixteen-week-old male CD1 mice were used for all our experiments. Thirty minutes before the immobilization procedure, 0.1 mg/kg of buprenorphine was administrated IP. The mice were then anesthetized using isoflurane. The right hindlimb was immobilized as previously described. Briefly, the hindlimb was immobilized 7 days by stapling the foot exploiting normal dorso-tibial flexion using an Autosuture Royal 35W skin stapler. One tine was inserted close to the toe at the plantar portion of the foot while the other was inserted in the distal portion of the gastrocnemius. The other hindlimb was used as a control. During the immobilization period, the mice were injected subcutaneously with MG132 (7.5 mg/kg/dose) or vehicle (DMSO) twice daily. DMSO containing or not MG132 was diluted in sterile pure corn oil (1:100, injected volume 150 μL). After 7 days, the tibialis anterior (TA) muscles of immobilized and non-i |
| 别名 | Z-LLL-al, Z-Leu-Leu-Leu-CHO, MG132 |
| 分子量 | 475.62 |
| 分子式 | C26H41N3O5 |
| CAS No. | 133407-82-6 |
| Smiles | CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)C=O |
| 密度 | 1.073 g/cm3 |
| 存储 | store at low temperature | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. | ||||||||||||||||||||||||||||||||||||||||
| 溶解度信息 | H2O: Insoluble Ethanol: 47.5 mg/mL (99.87 mM), Sonication is recommended. DMSO: 240 mg/mL (504.6 mM), Sonication is recommended. | ||||||||||||||||||||||||||||||||||||||||
| 体内实验配方 | 10% DMSO+40% PEG300+5% Tween 80+45% Saline: 9 mg/mL (18.92 mM), Suspension. 请按顺序添加溶剂,在添加下一种溶剂之前,尽可能使溶液澄清。如有必要,可通过加热、超声、涡旋处理进行溶解。工作液建议现配现用。以上配方仅供参考,体内配方并不是绝对的,请根据不同情况进行调整。 | ||||||||||||||||||||||||||||||||||||||||
溶液配制表 | |||||||||||||||||||||||||||||||||||||||||
Ethanol/DMSO
DMSO
| |||||||||||||||||||||||||||||||||||||||||
 化合物与蛋白结合的复合物
化合物与蛋白结合的复合物
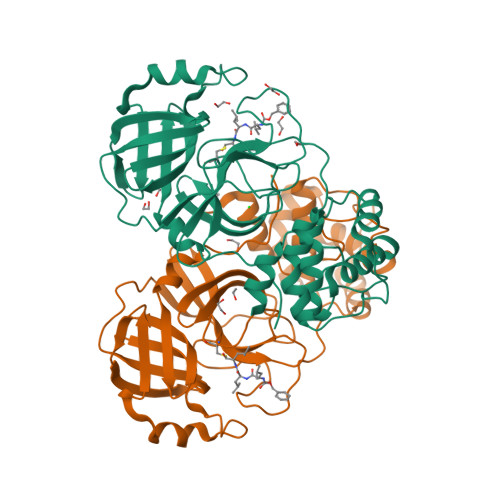
Crystal structure of MG-132 covalently bound to the main protease (3CLpro/Mpro) of SARS-CoV-2.
计算器
体内实验配液计算器
以上为“体内实验配液计算器”的使用方法举例,并不是具体某个化合物的推荐配制方式,请根据您的实验动物和给药方式选择适当的溶解方案。
剂量转换
对于不同动物的给药剂量换算,您也可以参考 更多




 很棒
很棒
 |
|