 您的购物车当前为空
您的购物车当前为空
(S)-Higenamine hydrobromide
一键复制产品信息(S)-Higenamine hydrobromide, an S-enantiomer of Higenamine, serves as the preliminary compound in benzylisoquinoline alkaloid biosynthesis. It is formed through the condensation of dopamine and 4-hydroxyphenylacetaldehyde (4-HPAA) via norcoclaurine synthase (NCS)[1].
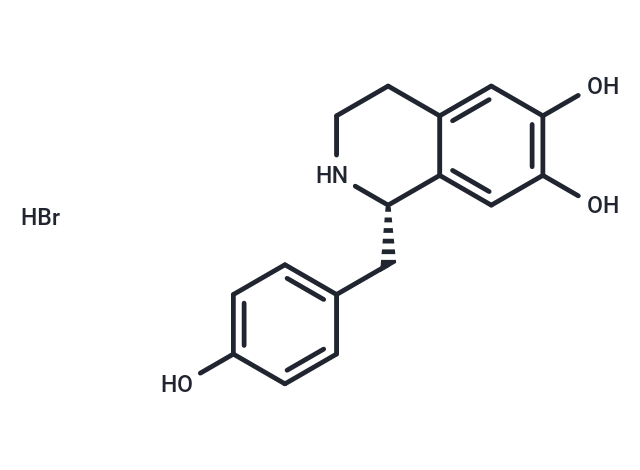
(S)-Higenamine hydrobromide
一键复制产品信息(S)-Higenamine hydrobromide, an S-enantiomer of Higenamine, serves as the preliminary compound in benzylisoquinoline alkaloid biosynthesis. It is formed through the condensation of dopamine and 4-hydroxyphenylacetaldehyde (4-HPAA) via norcoclaurine synthase (NCS)[1].
| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1 mg | ¥ 1,320 | 待询 |
产品介绍
| 产品描述 | (S)-Higenamine hydrobromide, an S-enantiomer of Higenamine, serves as the preliminary compound in benzylisoquinoline alkaloid biosynthesis. It is formed through the condensation of dopamine and 4-hydroxyphenylacetaldehyde (4-HPAA) via norcoclaurine synthase (NCS)[1]. |
| 体外活性 | The biosynthetic pathway leading to benzylisoquinoline alkaloids originates from the enzyme-catalyzed condensation of dopamine and 4-hydrophenylacetaldehyde to yield (S)-norcoclaurine. Both substrates are secondary metabolites derived from the decarboxylation/hydroxylation/deamination of tyrosine[1]. |
| 分子量 | 352.22 |
| 分子式 | C16H18BrNO3 |
| CAS No. | 105990-27-0 |
| Smiles | Br.Oc1ccc(C[C@@H]2NCCc3cc(O)c(O)cc23)cc1 |
| 存储 | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. |
计算器
体内实验配液计算器
剂量转换
对于不同动物的给药剂量换算,您也可以参考 更多





 还可以
还可以
 |
|