 您的购物车当前为空
您的购物车当前为空
巯基
巯基染料是一类与巯基(-SH)发生特异性反应的化学试剂,常用于修饰和标记含巯基的生物分子,如蛋白质或肽段。通过形成稳定的共价键,可赋予目标分子荧光、显色或其他功能性。广泛应用于生物成像、蛋白分析及药物开发领域。
- Fluorescein-5-maleimide荧光素-5-马来酰亚胺, N-(5-Fluoresceinyl)maleimideT1899175350-46-8Fluorescein-5-maleimide (N-(5-Fluoresceinyl)maleimide) 是硫醇反应荧光染料 (λex=494 nm,λem=519 nm),可用于将荧光素与蛋白质缀合。
- ¥ 297
规格数量 - NBD-X acidNBD Hexanoic Acid, C6-NBD, 6-(7-Nitrobenzofurazan-4-ylamino)hexanoic AcidT8697788235-25-0NBD-X acid是一种对环境敏感的荧光探针,用于研究脂质和蛋白质等生物聚合物,标记产量高于NBD,在水溶液中荧光强度显著降低。NBD-X acid能够发出绿色到黄色的荧光,激发/吸收波长为465/535 nm。
- ¥ 179
规格数量 - BromobimaneMonobromobimaneT1892971418-44-5Bromobimane (Monobromobimane) 是一种非荧光物质,但与硫醇反应反应时生成荧光产物。Bromobimane 可被当作一种探针使用,常在临床医学上检验血液中硫化物的含量。
- ¥ 145
规格数量 - 5-IAF5-碘乙酰氨基荧光素, 5-IodoacetamidofluoresceinT1405263368-54-75-IAF 是吲哚乙酰胺的一种荧光素衍生物,是一种荧光探针,可用来标记蛋白质和其他含有游离硫醇(半胱氨酸侧链)。5-IAF 可标记狗肾脏Na,K-ATP 酶的催化(α)亚单位。
- ¥ 253
规格数量 - TPE-MITetraphenylethene maleimideT742471245606-71-6TPE-MI (Tetraphenylethene maleimide) 是一种聚集诱导的发光原,可对活细胞进行染色,可用于捕捉露出的未折叠蛋白中的半胱氨酸,可检测双氢青蒿素研究疟疾寄生虫后的蛋白质损伤。
- ¥ 497
规格数量 - NBD-ClNBD chloride, 4-氯-7-硝基苯并-2-氧杂-1,3-二唑T1903010199-89-0NBD-Cl (NBD chloride) 是非荧光剂,能够与巯基或氨基反应,产生高度荧光。
- ¥ 99
规格数量 - ABD-F7-氟苯呋咱-4-硫氨, 4-(Aminosulfonyl)-7-fluoro-2,1,3-benzoxadiazoleT8386091366-65-3ABD-F(7-氟苯呋咱-4-硫氨)是一种高反应活性的荧光试剂,用于检测和定量含巯基的氨基酸、蛋白质及组织中的巯基,最大激发和发射波长分别为389 nm和513 nm。
- ¥ 186
规格数量 - BODIPY-TSThiol-green 2, BODIPY-TST392341808174-52-8BODIPY-TS (also known as Thiol-green 2) is a highly efficient thiol-specific turn-on probe with a fast response. It incorporates a thiosulfonate scaffold as its thiol recognition unit. BODIPY-TS displays excellent selectivity, low detection limit, and quantitative reactivity towards thiols. Moreover, it exhibits low toxicity. Notably, BODIPY-TS exhibits excitation at 490 nm and emission at 515 nm.
- ¥ 45300
规格数量 - Tetramethylrhodamine-6-maleimideT87505174568-68-4Tetramethylrhodamine-6-maleimide 是一种具有荧光特性的染料,包含与半胱氨酸共价偶联的活性巯基特异性部分。Tetramethylrhodamine-6-maleimide 可作为标记,用于实时检测全活性 Na+/K+-ATPase 的局部蛋白质运动。
- 待询
规格数量 - Dibromobimane二溴双马T8621168654-25-1Dibromobimane 是一种硫醇选择性荧光显像剂,用于交联含 cysteine 和 homocysteine 的肽。
- 待询
规格数量 - BDP 630/650 maleimideT858262183473-31-4BDP 630/650 maleimide, a fluorophore compatible with the Cyanine5 channel, is valuable for fluorescence lifetime measurements due to its extended excited state lifetime [1].
- 待询
规格数量 - 5MP-FluoresceinT854912100282-58-25MP-Fluorescein (compound 3e), a fluorescein dye derived from 5-Methylene pyrrolone (5MP), exemplifies a bioconjugation tool that is both highly thiol-specific and can be removed without a trace [1].
- 待询
规格数量 - Naph-EA-malThiol-green 1, Naph-EA-malT39547210292-65-2Naph-EA-mal (Thiol-green 1) is a highly efficient and quick-responding thiol fluorescence probe specifically designed for protein labeling and bioimaging applications. This compound offers a rapid detection method and exhibits ultrafast turn-on fluorescence upon interaction with thiols. Naph-EA-mal (Thiol-green 1) enables the detection of thiols in live cells, facilitates the labeling of protein thiols, enables quantification of total thiol concentration in cell lysate, and allows for the determination of reversible protein thiol oxidation in fixed cells. It possesses an excitation wavelength of 488 nM and an emission wavelength of 540 nM.
- ¥ 1670
规格数量 - N-(9-Acridinyl)maleimideT7843849759-20-8N-(9-Acridinyl)maleimide为一类马来酰亚胺荧光硫醇探针,本身不具较强荧光。当N-(9-Acridinyl)maleimide与含硫醇的化合物发生偶联反应时,会产生具有显著蓝色荧光的产物,适用于半胱氨酸和谷胱甘肽的荧光分析。
- ¥ 347
规格数量 - Cyanine3 maleimide tetrafluoroborateT861362755154-93-7Cyanine3 maleimide tetrafluoroborate是一种巯基特异性标记试剂,λex为550 nm,λem为580 nm,可用于蛋白质和肽标记。
- 待询
规格数量 - N-(3-Fluoranthenyl)maleimideN-(3-Fluoranthyl)顺丁烯二酰亚胺T8694160354-76-9N-(3-Fluoranthyl)maleimide, a thiol fluorescent probe with a lifetime of 20 nsec, has a maximum excitation wavelength of 370 nm and is suitable for studying time-dependent processes of biopolymers [1].
- 待询
规格数量 - N-4-(5,6-Dimethoxy-N-phthalimidinyl)phenylmaleimideT86951143503-03-1N-4-(5,6-Dimethoxy-N-phthalimidinyl)phenylmaleimide 是一种荧光试剂,主要应用于硫醇的标记。
- 待询
规格数量 - NIR-Thiol dinitrobenzenesulfonateT19039NIR-Thiol dinitrobenzenesulfonate has both absorption and emission in the NIR region. It is capable of imaging endogenously produced thiol in living cells and mice.
- ¥ 10600
规格数量



















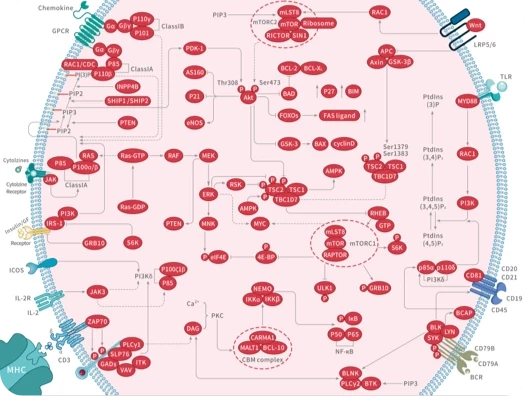
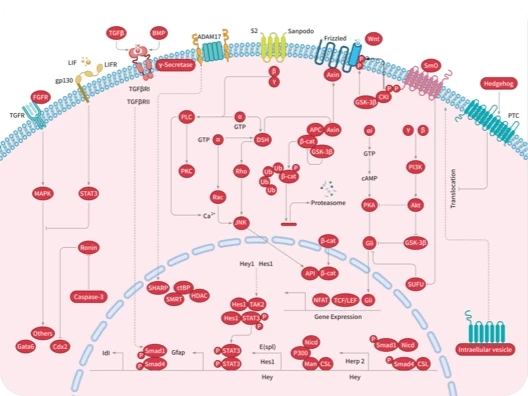
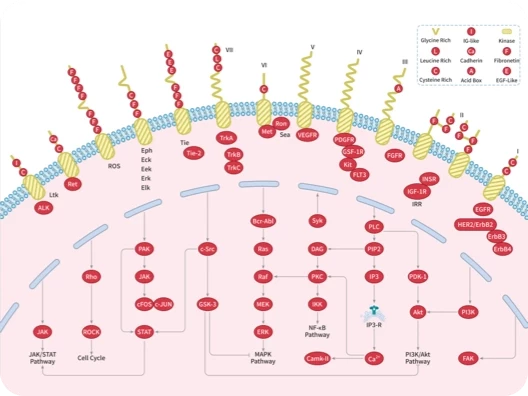
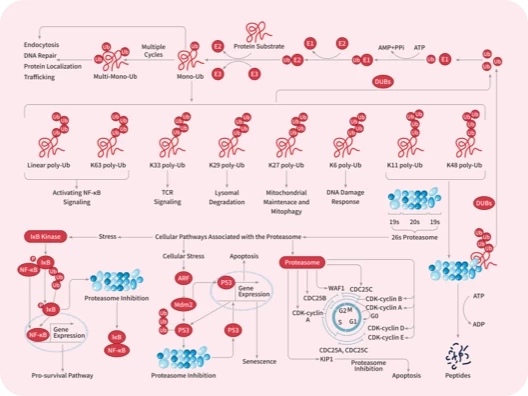

 |
|