 您的购物车当前为空
您的购物车当前为空
BODIPY系列
BODIPY系列染料是一类以硼二吡咯甲烷(Boron Dipyrromethene)为母核的荧光染料,具有优异的光稳定性、良好的水溶性和低毒性。通过化学修饰可实现对不同生物分子的特异性标记,广泛应用于细胞器成像、脂质追踪及活体检测等领域。其激发和发射波长范围广,适合多色成像实验。
- BODIPY 581/591 C11T39652217075-36-0In houseBODIPY 581/591 C11 是一种荧光放射探针,用于指示模型膜系统和活细胞中的脂质过氧化和抗氧化功效。
- ¥ 1980
规格数量
 客户已引用
客户已引用 - BODIPY 493/503Pyrromethene 546, BDP 493/503 lipid stainT36957121207-31-6BODIPY 493/503 (Pyrromethene 546) 属于亲脂性荧光染料,Ex/Em 为 493/503 nm。 BODIPY 493/503 定位于极性脂质,可用于标记细胞中性脂质含量以及活细胞和固定细胞应用。
- ¥ 413
规格数量 客户已引用
客户已引用 - Py-BODIPY-NHS esterNanoBRET 590-SET18987201998-61-0Py-BODIPY-NHS ester 是一种具有强紫外吸收能力的小分子染料,可用于标记活细胞和固定细胞。
- ¥ 256
规格数量 - BODIPY 505/515T3695821658-70-8BODIPY 505/515 是一种亲脂性荧光探针,可定位到细胞内的脂质体,已被用于标记脂滴。BODIPY 505/515 已被用于电子显微镜、外荧光显微镜、共聚焦显微镜以及各种藻类物种的流式细胞仪应用中。
- ¥ 108
规格数量 - BODIPY 630/650XT82844380367-48-6BODIPY 630/650X是腺苷受体配体 N-乙基羧酰胺腺苷 (NECA) 的荧光标记偶联物,其激发和发射峰分别位于 630 nm和 650 nm,BODIPY 630/650是靶向腺苷受体活性与分布的荧光检测、成像的重要工具。
- ¥ 1980
规格数量 - BODIPY-FLBDP FL acidT66518165599-63-3BODIPY-FL (BDP FL acid) 是一种广谱且有效的荧光染料,可用于标记探针或引物,是一种基于荧光猝灭的定量检测特定 DNA/RNA 的化合物。BODIPY-FL 标记的单萜可用于检测革兰氏阳性和革兰氏阴性细菌的特征和致病真菌。
- ¥ 108
规格数量 - BODIPY FL prazosinT63980175799-93-6In houseBODIPY FL prazosin 是一种高效的 α1-肾上腺素拮抗剂,抑制 α1a-AR 和 α1b-AR. BODIPY FL prazosin 是一种荧光配体(λEx(nm)/ λEm (nm): 485/535),常与流式细胞术或共聚焦激光扫描显微镜一起研究α1-肾上腺素亚型和细胞成像。
- ¥ 1980

规格数量 - ¥ 1980
- 8-Phenyl-BODIPY 505/515T78449194235-40-08-Phenyl-BODIPY 505/515是苯基取代的 BODIPY 衍生物,从而改变BODIPY的磷光性质、三重态和单线态氧生成,可以用作荧光探针。
- ¥ 122
规格数量 - 8-(4-iodophenyl)-1,3,5,7-tetramethyl BODIPYT73588250734-47-58-(4-iodophenyl)-1,3,5,7-tetramethyl BODIPY 是一种新型的 BODIPY 荧光染料,可用于治疗细胞毒性研究。
- ¥ 158
规格数量 - 8-Phenyl-2,6-dibromo-1,3,5,7-tetramethyl BODIPYT73590910823-84-68-Phenyl-2,6-dibromo-1,3,5,7-tetramethyl BODIPY 可用于光动力疗法。
- ¥ 198
规格数量 - BODIPY-CholesterolBCh2T41008878557-19-8BODIPY-Cholesterol (TF-Chol)是一种带有荧光 BODIPY 基团和细胞通透性的胆固醇类似物,是一种新型 BODIPY 胆固醇探针。BODIPY-Cholesterol 可用于研究细胞内固醇摄取和细胞器间固醇通量。
- ¥ 1864
规格数量 - 8-Chloro-1,3,5,7-tetramethyl BODIPYT2058311414345-95-18-Chloro-1,3,5,7-tetramethyl BODIPY是一种荧光染料,其化学结构以氟硼二吡咯为核心,并在吡咯环的特定位点被甲基和氯替代。该化合物具有较高的荧光量子产率和特定的吸收、发射波长。Ex/Em(nm)=500/515。
- ¥ 1300
规格数量 - BODIPY558/568 carboxylic acidT205826150173-72-1BODIPY 558/568 carboxylic acid拥有高量子产率和消光系数,表现出明亮的黄红色荧光。Ex/Em(nm)=558/568。
- ¥ 1430
规格数量 - BODIPY-TSThiol-green 2, BODIPY-TST392341808174-52-8BODIPY-TS (also known as Thiol-green 2) is a highly efficient thiol-specific turn-on probe with a fast response. It incorporates a thiosulfonate scaffold as its thiol recognition unit. BODIPY-TS displays excellent selectivity, low detection limit, and quantitative reactivity towards thiols. Moreover, it exhibits low toxicity. Notably, BODIPY-TS exhibits excitation at 490 nm and emission at 515 nm.
- ¥ 45300
规格数量 - BODIPY FL VH032T411712770675-66-4BODIPY FL VH032 is a high-affinity VHL fluorescent probe (Kd= 3.01 nM) with application in time-resolved fluorescence resonance energy-transfer (TR-FRET) assays for high-throughput identification and characterization of VHL ligands. BODIPY FL VH032 consists of a derivative of von Hippel-Lindau (VHL) ligand VH 032 and a fluorophore BODIPY FL(with excitation peak at 504 nm and emission peak at 520 nm), joined by a polyethylene glycol (PEG) 4 linker. The BODIPY FL VH032 VHL TR-FRET signal is stable through the 90 - 300 min incubation time, and theTR-FRET binding assay is sensitive, selective and resistant to assay interference. BODIPY FL VH032 also exhibits high VHL affinity in a fluorescence polarization (FP) assay. Excitation maximum ~ 504 nm; emission maximum ~ 520 nm.
- ¥ 1300
规格数量 - Caffeine orangeT859941632296-13-9Compound 1, known as Caffeine Orange, serves as a highly selective aqueous-phase fluorescence turn-on sensor specifically for caffeine. When exposed to 532 nm green excitation light, this compound causes caffeinated coffee to turn orange. Caffeine Orange boasts impressive photophysical properties, including a high extinction coefficient, excellent light stability, and a narrow emission bandwidth, making it valuable in the development of caffeine detection devices [1].
- 待询
规格数量 - BODIPY 665/676T85875164106-16-5BODIPY 665/676 is a lipophilic, radical-sensitive fluorescent probe utilized to investigate radical-driven lipid autoxidation [1].
- ¥ 2480
规格数量 - BDP 581/591 amine hydrochlorideBDP 581/591 amineT858212183472-97-9BDP 581/591 amine hydrochloride 是一种 BODIPY 染料连接剂,作为通用的、光稳定的荧光团。其胺基团使该化合物能够与羧酸、活化的 NHS 酯和其他羰基发生反应。
- ¥ 1430
规格数量 - 5-Carboxyrhodamine 6G succinimidyl esterR6G-SET85482209112-21-05-Carboxyrhodamine 6G succinimidyl ester (R6G-SE)是一种胺反应性荧光染料,具有显著的标记功能。
- 待询
规格数量 - Bodipy TR alkyneT858892006345-35-1Bodipy TR alkyne是一类硼二吲哚亚甲基 (BDI) 染料,适用于TAMRA通道,常用于寡核苷酸标记和氨基酸测序。
- 待询
规格数量 - BODIPY 505/515-8-C3-COOHT85871878674-84-1BODIPY 505/515-8-C3-COOH,一种绿色荧光分子,广泛用作纳米绿藻脂滴成像的荧光染料。此外,该化合物亦适用于通过流式细胞术进行高通量筛选及细胞分选的研究应用。
- 待询
规格数量 - BODIPY-581/591 NHS esterT85893150152-70-8BODIPY-581/591 NHS ester是一种明亮的红色荧光染料 (excitation: 581 nm; emission: 591 nm),具有独特的疏水特性,可对脂质、膜和其他亲脂化合物染色。
- ¥ 892
规格数量 - Pyrromethene 597T78457137829-79-9Pyrromethene 597 是一种具有宽波长调谐范围及高光稳定性的 BODIPY 激光染料,可作为热探针使用。
- ¥ 218
规格数量



















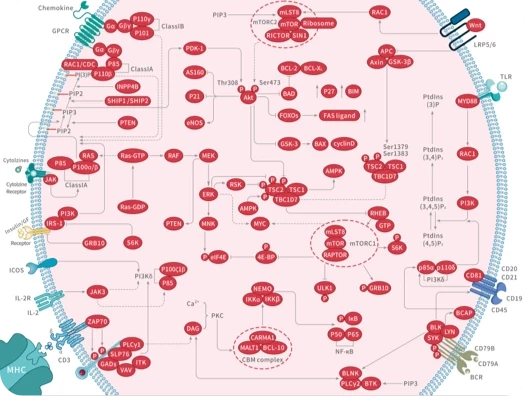
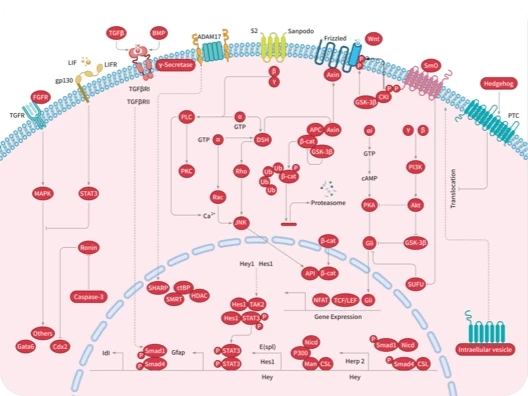
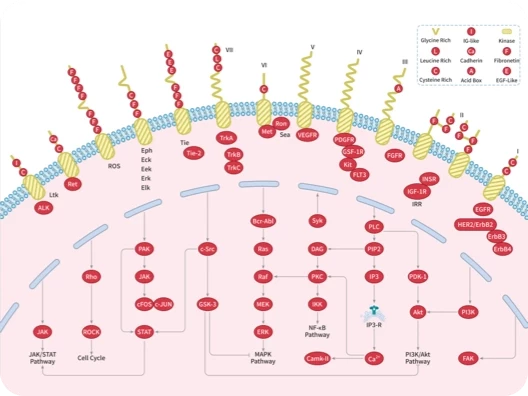
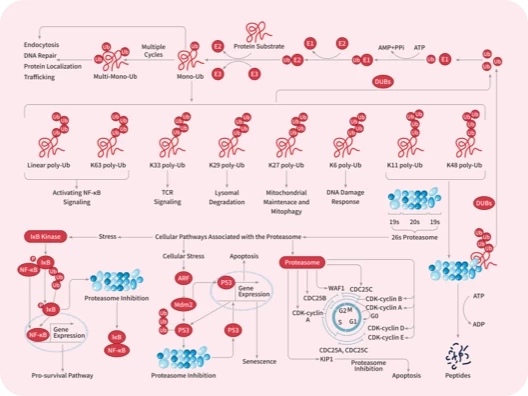

 |
|