 您的购物车当前为空
您的购物车当前为空
Cisplatin
一键复制产品信息别名 顺铂, cis-Diaminodichloroplatinum, CDDP
Cisplatin(CDDP)是一种具有抗肿瘤活性的化疗药物,是典型的DNA交联剂,能够通过在癌细胞中形成DNA加合物,抑制DNA合成并引起DNA损伤,从而导致细胞死亡。除此之外,Cisplatin 还可激活铁死亡(ferroptosis)并诱导自噬(autophagy)。在动物实验中,Cisplatin 常被用于构建慢性肾损伤和急性肾衰竭模型。
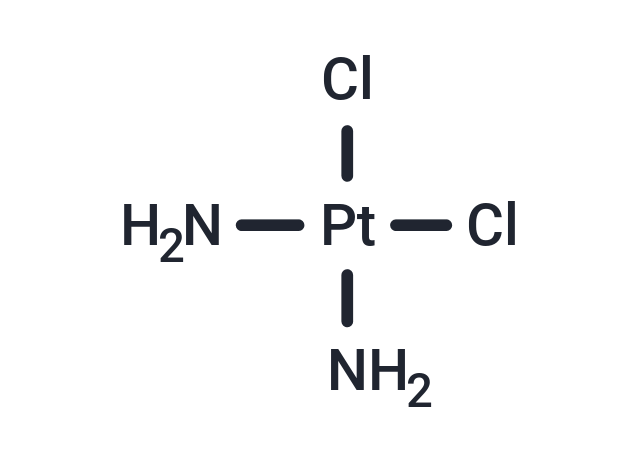

为众多的药物研发团队赋能,
让新药发现更简单!
Cisplatin
一键复制产品信息Cisplatin(CDDP)是一种具有抗肿瘤活性的化疗药物,是典型的DNA交联剂,能够通过在癌细胞中形成DNA加合物,抑制DNA合成并引起DNA损伤,从而导致细胞死亡。除此之外,Cisplatin 还可激活铁死亡(ferroptosis)并诱导自噬(autophagy)。在动物实验中,Cisplatin 常被用于构建慢性肾损伤和急性肾衰竭模型。
| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mg | ¥ 116 | 现货 | |
| 50 mg | ¥ 232 | 现货 | |
| 100 mg | ¥ 348 | 现货 | |
| 500 mg | ¥ 918 | 现货 | |
| 1 g | ¥ 1,543 | 现货 |
产品介绍
| 产品描述 | Cisplatin (CDDP) is a chemotherapeutic agent with antitumor activity and is a classic DNA crosslinker. It inhibits DNA synthesis and induces DNA damage in cancer cells by forming DNA adducts, ultimately leading to cell death. In addition, Cisplatin can activate ferroptosis and induce autophagy. In animal studies, it is commonly used to establish models of chronic kidney injury and acute renal failure. |
| 靶点活性 | SK-MES-1 cells:4.09 µM, MCF-7 cells:1.2 µM, A549 cells:1.58 µM, HCC38 cells:2.5 µM |
| 体外活性 | 方法:人非小细胞肺癌细胞 A549、SKMES-1、MOR 和 H460 用 Cisplatin (0.001-100 μM) 处理 72 h,使用 MTT 方法检测细胞生长抑制情况。 |
| 体内活性 | 方法:为检测体内抗肿瘤活性,将 Cisplatin (5 mg/kg/6 天) 和 Chloroquine (13 mg/kg/天) 腹腔注射给携带咽下鳞状细胞癌肿瘤 (HSCC) FaDu 的 BALB/c nude 小鼠,持续十八天。 |
| 细胞实验 | Rabbit renal proximal tubules were isolated using the iron oxide perfusion method and grown in 35-mm tissue culture dishes under improved conditions as described previously. The cell culture medium was a 1:1 mixture of Dulbecco's modified Eagle's medium/Ham's F-12 (without D-glucose, phenol red, or sodium pyruvate) supplemented with 15 mM HEPES buffer, 2.5 mM L-glutamine, 1 μM pyridoxine HCl, 15 mM sodium bicarbonate, and 6 mM lactate. Hydrocortisone (50 nM), selenium (5 ng/ml), human transferrin (5 μg/ml), bovine insulin (10 nM), and L-ascorbic acid-2-phosphate (50 μM) were added to fresh culture medium immediately before daily media change. In general, confluent RPTCs were treated with inhibitors or diluent control [typically DMSO at 0.1% (v/v)] for 30 min before treatment with cisplatin. Aliquots of RPTCs were used for various assays as detailed below [1]. |
| 动物实验 | Mice were divided randomly into three groups (Control, Cisplatin and Cisplatin+HemoHIM), and each group consisted of twenty mice. B16F0 melanoma (5 × 10^5 cells/mouse) was inoculated into subcutaneous femoral left region of mice at 3 days before an initial injection of cisplatin. Cisplatin was injected intraperitoneally at 4 mg/kg body weight (B.W.) on day 0, 7 and 14 (total three injections). Experimental group was intubated with HemoHIM at a final concentration of 100 mg/kgB.W. by everyday from day -1 to day 16, while the control group received only water. On day 17 after initial injection of cisplatin, all mice of each group were experimented, respectively, to evaluate tumor weight or tumor size. The tumor size was calculated as follows: tumor size = ab^2/2, where a and b are the larger and smaller diameters, respectively [3]. |
| 别名 | 顺铂, cis-Diaminodichloroplatinum, CDDP |
| 分子量 | 300.04 |
| 分子式 | Cl2H6N2Pt |
| CAS No. | 15663-27-1 |
| Smiles | N[Pt](Cl)(Cl)N |
| 密度 | 3.7 |
| 存储 | keep away from direct sunlight,The compound is unstable in solution. Please use soon | Powder: -20°C for 3 years | Shipping with blue ice/Shipping at ambient temperature. | |||||||||||||||||||||||||||||||||||
| 溶解度信息 | H2O: 1 mg/mL (3.3 mM), Heating to 50℃ is recommended. The compound is unstable in solution. Please use soon.(DMSO can inactivate Cisplatin's activity.) DMF: 20 mg/mL (66.66 mM), Sonication is recommended. The compound is unstable in solution. Please use soon. | |||||||||||||||||||||||||||||||||||
溶液配制表 | ||||||||||||||||||||||||||||||||||||
H2O/DMF
DMF
| ||||||||||||||||||||||||||||||||||||
 化合物与蛋白结合的复合物
化合物与蛋白结合的复合物
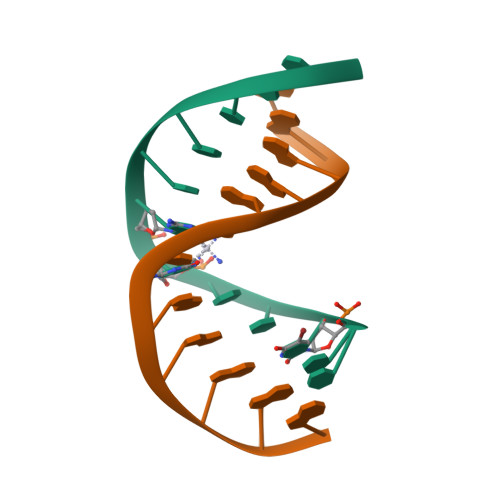
Solution Structures of a DNA Dodecamer Duplex with a Cisplatin 1,2-d(GG) Intrastrand Cross-Link
计算器
体内实验配液计算器
以上为“体内实验配液计算器”的使用方法举例,并不是具体某个化合物的推荐配制方式,请根据您的实验动物和给药方式选择适当的溶解方案。
剂量转换
对于不同动物的给药剂量换算,您也可以参考 更多




 很棒
很棒
 |
|