 您的购物车当前为空
您的购物车当前为空
Deleobuvir sodium
一键复制产品信息Deleobuvir sodium is the salt form of Deleobuvir, also known as BI207127, a non-nucleoside hepatitis C virus NS5B polymerase inhibitor for the treatment of hepatitis C. Deleobuvir was tested in combination regimens with pegylated interferon and ribavirin, and in interferon-free regimens with other direct-acting antiviral agents including faldaprevir. Deleobuvir showed that a triple combination of deleobuvir, faldaprevir, and ribavirin performed well in HCV genotype 1b patients. Efficacy fell below 50%, however, for dual regimens without ribavirin and for genotype 1a patients. In December 2013, deleobuvir was discontinued since recent findings from phase III trials did not suggest sufficient efficacy.
Deleobuvir sodium
一键复制产品信息Deleobuvir sodium is the salt form of Deleobuvir, also known as BI207127, a non-nucleoside hepatitis C virus NS5B polymerase inhibitor for the treatment of hepatitis C. Deleobuvir was tested in combination regimens with pegylated interferon and ribavirin, and in interferon-free regimens with other direct-acting antiviral agents including faldaprevir. Deleobuvir showed that a triple combination of deleobuvir, faldaprevir, and ribavirin performed well in HCV genotype 1b patients. Efficacy fell below 50%, however, for dual regimens without ribavirin and for genotype 1a patients. In December 2013, deleobuvir was discontinued since recent findings from phase III trials did not suggest sufficient efficacy.
| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25 mg | ¥ 15,000 | 8-10周 | |
| 50 mg | ¥ 19,800 | 8-10周 | |
| 100 mg | ¥ 25,500 | 8-10周 |
产品介绍
| 产品描述 | Deleobuvir sodium is the salt form of Deleobuvir, also known as BI207127, a non-nucleoside hepatitis C virus NS5B polymerase inhibitor for the treatment of hepatitis C. Deleobuvir was tested in combination regimens with pegylated interferon and ribavirin, and in interferon-free regimens with other direct-acting antiviral agents including faldaprevir. Deleobuvir showed that a triple combination of deleobuvir, faldaprevir, and ribavirin performed well in HCV genotype 1b patients. Efficacy fell below 50%, however, for dual regimens without ribavirin and for genotype 1a patients. In December 2013, deleobuvir was discontinued since recent findings from phase III trials did not suggest sufficient efficacy. |
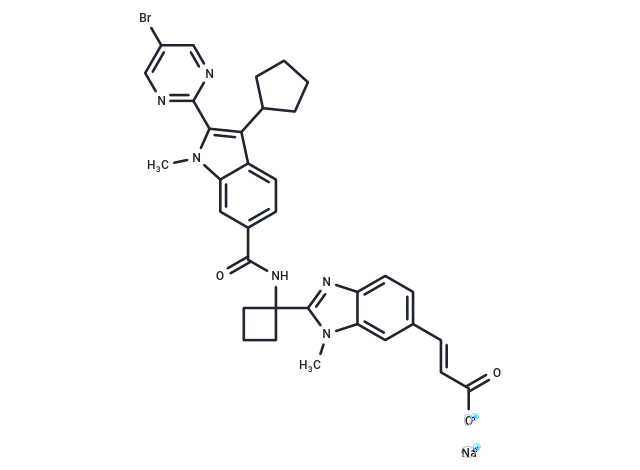
| 分子量 | 675.56 |
| 分子式 | C34H32BrN6NaO3 |
| CAS No. | 1370023-80-5 |
| Smiles | [Na+].Cn1c(c(C2CCCC2)c2ccc(cc12)C(=O)NC1(CCC1)c1nc2ccc(\C=C\C([O-])=O)cc2n1C)-c1ncc(Br)cn1 |
| 存储 | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. |
计算器
体内实验配液计算器
以上为“体内实验配液计算器”的使用方法举例,并不是具体某个化合物的推荐配制方式,请根据您的实验动物和给药方式选择适当的溶解方案。
剂量转换
对于不同动物的给药剂量换算,您也可以参考 更多





 还可以
还可以
 |
|