 您的购物车当前为空
您的购物车当前为空
Traditional drug research and development are mainly based on natural active products or screening new drugs from existing compound data, but this method is highly random, blind, and inefficient. Medicinal chemists subsequently developed high-throughput screening (HTS) methods for drug discovery. Many pharmaceutical companies have also established compound libraries containing millions of small molecules and discovered many drug candidates. However, in drug screening with complex targets, HTS has been repeatedly frustrated. It is difficult to screen high-potential compounds, or the screened compounds have high false positives and poor drug-like properties. In this context, Fragment-based drug design (FBDD) came into being.
The theoretical basis of FBDD is to select favorable fragment combinations or extensions to obtain new drug molecules, with a higher probability of obtaining highly active drug candidates. Compared with the screening of millions of macromolecules, thousands of fragment molecules can be combined to form millions of drug structures, which are easier to collect and manage. In addition, fragments have smaller molecular weights, relatively higher solubility, and easier structural optimization. The potential of over-the-counter medicine is higher. The high solubility multifunctional fragment library is designed by analyzing the spatial stability of all commercially available fragments and selecting a chemically diverse subset. In order to enhance the diversity of the compound library, we carefully selected some additional saturated heterocyclic compounds to supplement the compound library.





 很棒
很棒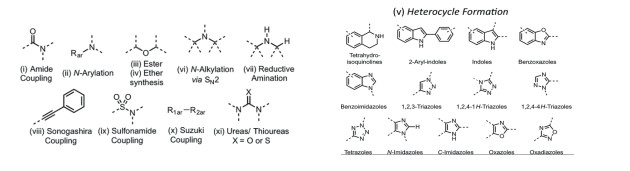

 |
|